Additional information
| Use in sports | None, not a performance-enhancing drug |
|---|---|
| Formula | C22H30N6O2 |
| Side effects | Hypoglycemia, headache, nasopharyngitis, upper respiratory tract infection |
| Effects | Improves blood sugar control in type 2 diabetes |
| Dosage (sports) | Not applicable as not used for enhancing athletic performance |
| Dosage (medical) | Typically 20 mg once daily |
| Half-life | 24 hours approximately |
| Main action | Antidiabetic, increases insulin release and decreases glucagon levels |
| Substance class | Dipeptidyl peptidase-4 (DPP-4) inhibitor |
| Chemical name | (2S)-2-[[4-[(3-methyl-1-phenyl-1H-pyrazol-5-yl)amino]pyrimidin-2-yl]amino]butanoic acid |
| Water Retention | No significant water retention |
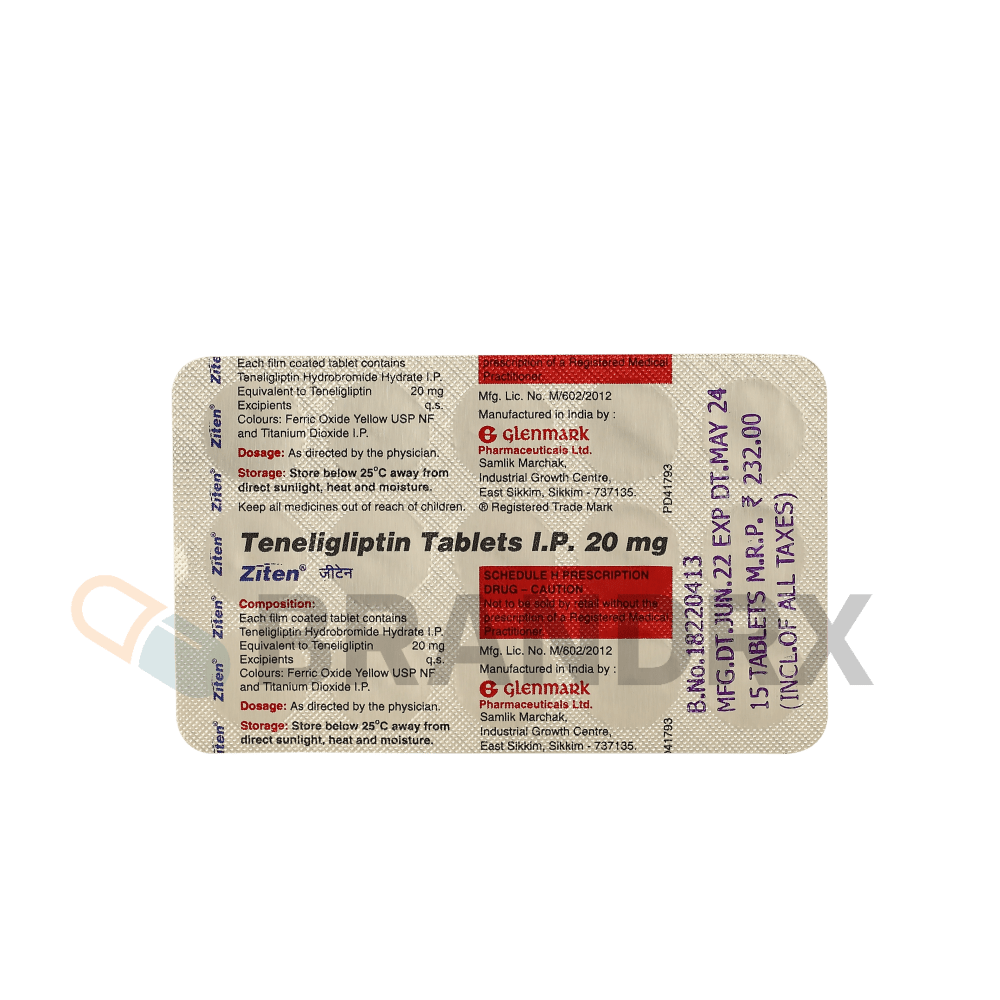
| Storage conditions | Store at room temperature, away from moisture and heat |
| Trade name | Tenelia |
| Blood pressure | Can slightly reduce blood pressure |
| Also known as | Tenelia, Teneglucon |
| Strength | 20 mg |
| Lab Test | Monitoring blood glucose levels |
| Hepatotoxicity | Low risk |
| Manufacturer | Glenmark Pharmaceuticals Ltd. |